

Targeted Integration: Goepfert pointed out that the most significant change made to Roche’s cell line development process in the past three years was switching from random to targeted integration. New technologies and approaches are being used to meet those challenges. Biopharmaceutical developers also are facing increasing pressure to proceed quickly to clinical trials. Ulrich Goepfert (principal scientist, cell technologies, at the Roche Innovation Center) noted that cell line development has become increasingly challenging with the emergence of sophisticated biologics such as complex engineered BsAbs, trivalent T-cell engagers, tumor-targeted checkpoint modulators, and antibody cytokine fusions. Monday’s presentations began with two keynote addresses, the first of which focused on current CLD trends. And new approaches are helping scientists to study genes that are directly related to cell production, quality, and viability.
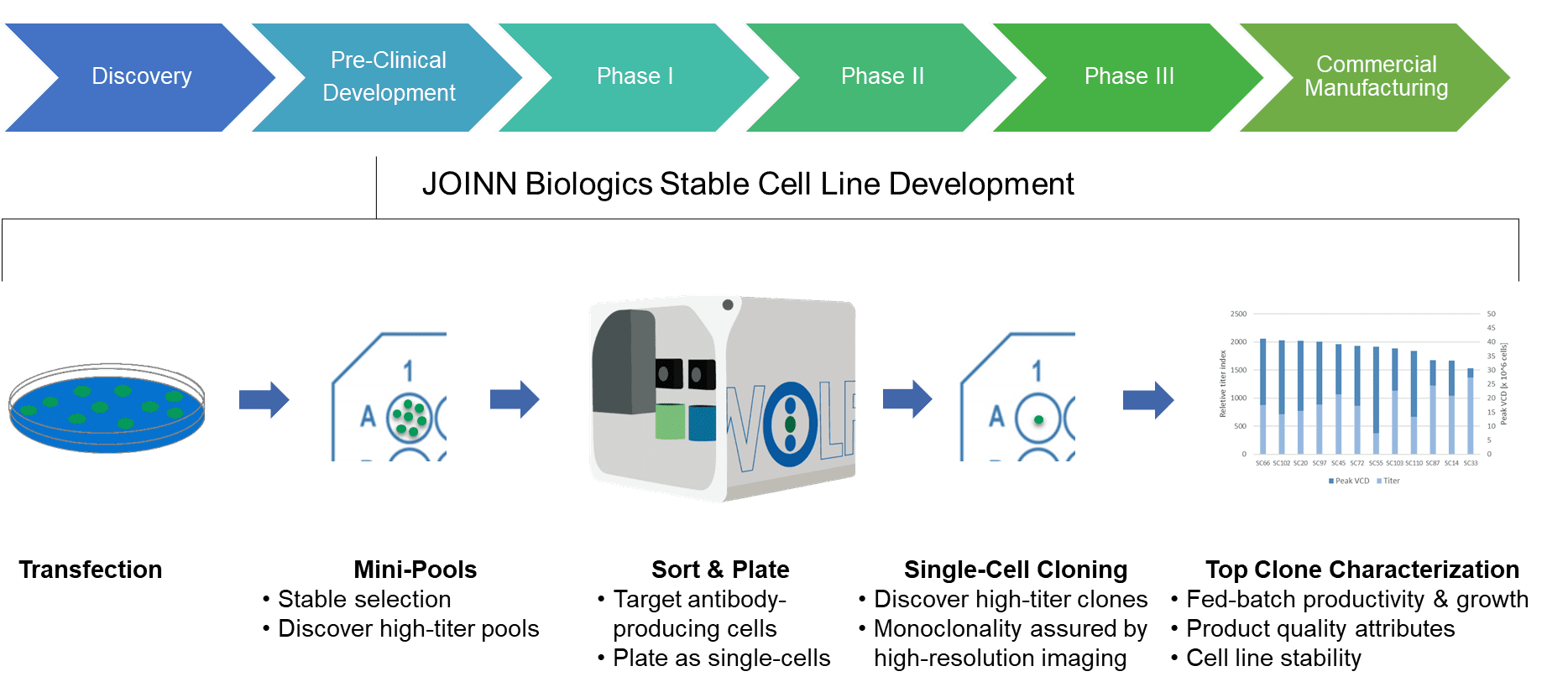
Online presentations from the CLD track of the BioProcess International Europe conference, which was held 13–17 July 2020, addressed current implementations of high-throughput screening, synthetic biology, genetic engineering, next- generation sequencing, and automated analysis. Researchers are creating advanced strategies and tools to overcome those challenges, especially for complex biologics such as bispecific antibodies (BsAbs) and difficult-to-express (DTE) proteins. However, cell line development (CLD) can be tedious and time-consuming work, and every stage in the CLD workflow has its limitations and challenges. For a host-cell system to generate high yields of recombinant proteins and other entities, cells must be derived from optimized and stable cell lines.


 0 kommentar(er)
0 kommentar(er)
